Locally Advanced and Metastatic Pancreatic Cancer
“In K-Ras Wild-type locally Advanced or Metastatic Pancreatic Cancer, Nimotuzumab + Gemcitabine regime improved mOS compared with Placebo plus Gemcitabine, with a decrease of 50% mortality risk. [1]”
Demonstrated a notably extended median overall survival (mOS)
Nimotuzumab + Gemcitabine
10.9 months
vs
Placebo + Gemcitabine
8.5 months
(HR=0.50; 95% CI; 0.06-0.94; P=0.025)
mOS = median overall survival; HR = hazard ratio; CI = confidence interval
Demonstrated a notably extended progression free survival (PFS)
Nimotuzumab + Gemcitabine
4.2 months
vs
Placebo + Gemcitabin
3.6 months
(HR=0.56; 95% CI; 0.12-0.99; P=0.013)
mOS = median overall survival; HR = hazard ratio; CI = confidence interval
Nimotuzumab for Locally Advanced or Metastatic Pancreatic Cancer
Below is a summary of Phase III clinical trials of Nimotuzumab in pancreatic cancer , conducted by Qin, S. et al. (2022) and titled “Nimotuzumab Combined with Gemcitabine versus Placebo plus Gemcitabine in K-Ras Wild-type locally Advanced or Metastatic Pancreatic Cancer: A Prospective, Randomized-controlled, Double-blinded, Multicenter and Phase III Clinical Trial (NOTABLE Study).” [1]
Study Background
As a result of the favorable results observed in Phase II clinical trials with the Nimotuzumab and Gemcitabine combination, the study is advancing to Phase III.
Read More
Pancreatic cancer is a highly lethal malignancy with a significant global impact, recording approximately 495,773 new cases and 466,003 deaths in 2020. Alarmingly, 80% of pancreatic cancer cases are diagnosed in advanced stages.
Current treatment approaches yield unsatisfactory results, with a median overall survival (mOS) ranging from 6-8 months. Locally advanced cases offer a slight improvement at 6–9 months, while metastatic cases have a dismal mOS of 3-5 months. The 5-year survival rate is a mere 7-9%. 2
Despite significant advances in cancer treatment, pancreatic cancer has seen limited progress. K-Ras mutations, found in approximately 90% of cases in the United States and 87% in China, have posed a major challenge.
Nimotuzumab, a humanized anti-EGFR monoclonal antibody, holds promise by disrupting EGFR signaling pathways, inducing immune effects, and having the potential to be an effective treatment option for advanced pancreatic cancer.
In a phase II clinical trial conducted in Germany (designated as PCS07), it was observed that the combination of Nimotuzumab and gemcitabine, in comparison to a placebo in conjunction with gemcitabine, led to a statistically significant improvement in median overall survival (mOS), with respective values of 8.6 months and 6.0 months, particularly notable in the subgroup of patients with K-ras wild type, where the mOS extended to 11.62 months compared to 5.67 months in the control group. Moreover, the one-year survival rates were found to be 53.8% and 15.8% for the experimental and control groups, respectively.3
In both Phase II and Phase III clinical trials, the treatment of pancreatic cancer with Nimotuzumab + Gemcitabine demonstrated a superior mOS compared to the use of Gemcitabine alone.
Study Design
The present phase III trial (NOTABLE study) aims to further evaluate the efficacy and safety of Nimo plus Gem for treatment of K-Ras wide type locally advanced or metastatic PC.
A prospective study program that uses molecular targeted drug combined with chemotherapy drug for the enriched PC population by pre-screen K-Ras oncogene.

Primary endpoint: mOS
Secondary endpoints: PFS, TTP, ORR,DCR.CBR & Safety
* OS, overall survival; PFS, progression-free survival; TTP, time to disease progression; ORR, objective response rate; DCR, disease control rate, CBR, clinical benefit response
Efficacy
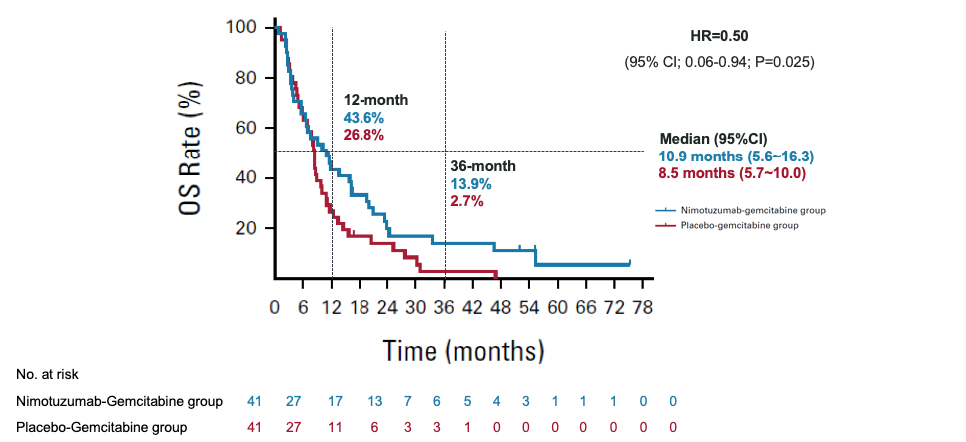
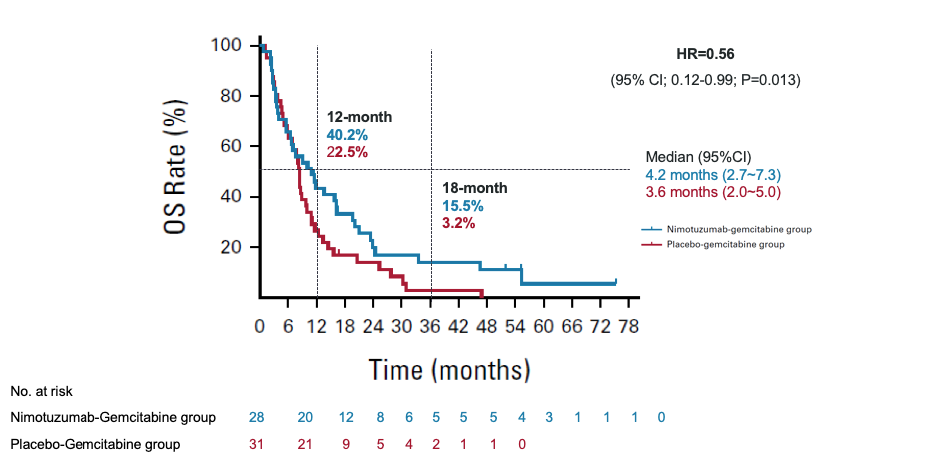
* There was a violation of the proportional hazards (PH) because the two survival curves cross. Restricted Mean Survival Time (RMST) method (RMSTREG procedure, log-linear models) was used to estimate hazard risk. The adjusted HR with 95% CI was used as primary estimate of the difference between the arms, stratified by tumor location, previous surgery history, previous treatment of bile obstruction, previous adjuvant chemotherapy history at baseline. Data cut-off, Nov.23,2021.
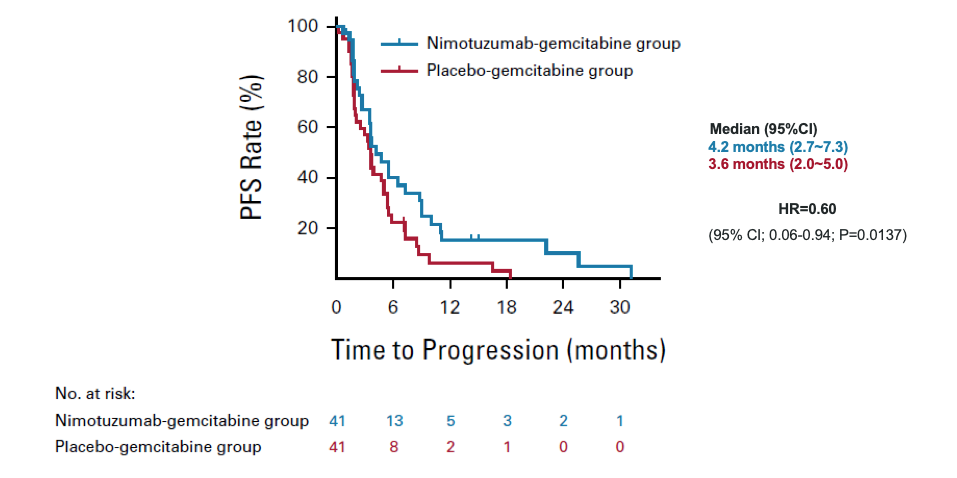
*Treatment arms were compared using log-rank tests stratified by tumor location, previous surgery history, previous treatment of bile obstruction, previous adjuvant chemotherapy history at baseline. The adjusted HR with 95% CI was used as the primary estimate of the difference between the arms. Data cut-off, Nov.23,2021
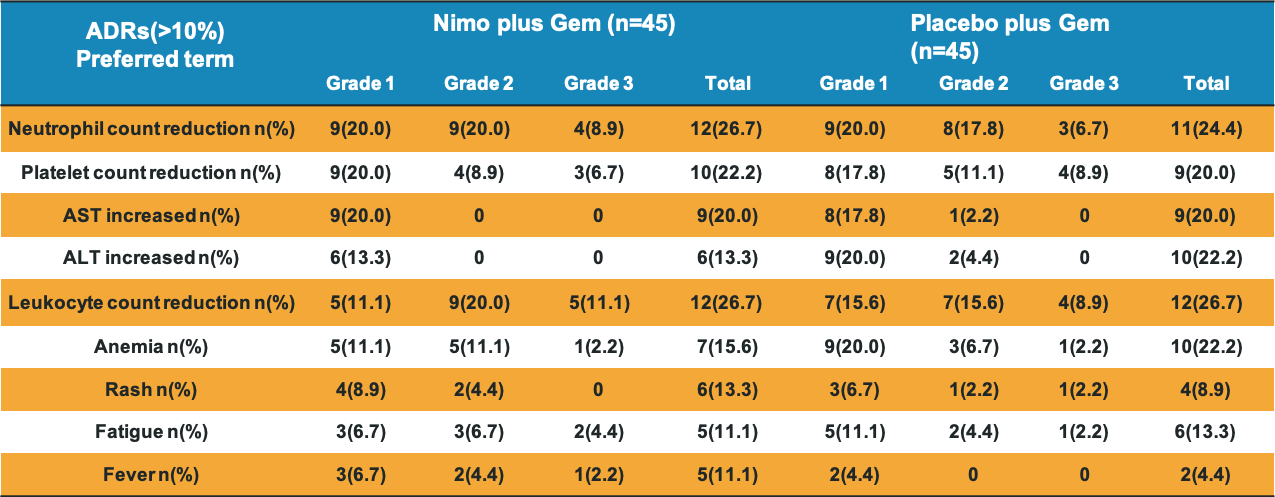
Safety Profile
Below is a summary of Phase III clinical trials of Nimotuzumab in pancreatic cancer , conducted by Qin, S. et al. (2022) and titled “Nimotuzumab Combined with Gemcitabine versus Placebo plus Gemcitabine in K-Ras Wild-type locally Advanced or Metastatic Pancreatic Cancer: A Prospective, Randomized-controlled, Double-blinded, Multicenter and Phase III Clinical Trial (NOTABLE Study).” [1]
Conclusion
Nimotuzumab combined with gemcitabine increases OS and PFS in patients with K-Ras wild-type locally advanced or metastatic pancreatic cancer. The safety profile of nimotuzumab is similar to placebo.
Reference
- Qin S, Li J, Bai Y, et al. Nimotuzumab Plus Gemcitabine for K-Ras Wild-Type Locally Advanced or Metastatic Pancreatic Cancer [published online ahead of print, 2023 Aug 30]. J Clin Oncol. 2023;JCO2202630. doi:10.1200/JCO.22.02630
- Cui J, Jiao F, et al. Chinese Society of Clinical Oncology (CSCO): Clinical guidelines for the diagnosis and treatment of pancreatic cancer. Journal of the National Cancer Center. 2022; 2. 10.1016/j.jncc.2022.08.006.
- Schultheis B, Reuter D, Ebert MP, et al. Gemcitabine combined with the monoclonal antibody nimotuzumab is an active first-line regimen in KRAS wildtype patients with locally advanced or metastatic pancreatic cancer: a multicenter, randomized phase IIb study. Ann Oncol. 2017;28(10):2429-2435. doi:10.1093/annonc/mdx343
